Results for 'clear mission'

New Book Unites Oncology’s Brightest Minds To Innovate Cancer Cures
Sep 9th • 5 mins read




“Extreme Interviewing” – MSL Interview Tips and Insights from Medical Affairs Leaders
Jul 9th • 1 min read



MSL Hiring and Recruitment: 5 Ways to Support Diversity and Inclusion
May 19th • 2 mins read


Presentation Nails and Fails: 7 Tips to Ace Your Next MSL Presentation
Oct 12th • 1 min read




Comparative study on anticancer drug access times between FDA, EMA and the French temporary authorisation for use program over 13 years
Apr 7th • 12 mins read

Assessment of Coverage in England of Cancer Drugs Qualifying for US Food and Drug Administration Accelerated Approval
Feb 22nd • 10 mins read

Assessment of Food and Drug Administration- and European Medicines Agency-Approved Systemic Oncology Therapies and Clinically Meaningful Improvements in Quality of Life: A Systematic Review
Feb 11th • 4 mins read
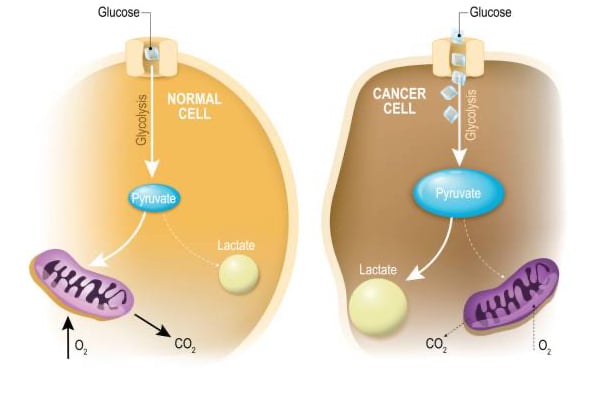
“Oncometabolism: The switchboard of cancer: An editorial”
Feb 1st • 1 min read

Safeguarding cancer research funding by European charities amidst the COVID-19 pandemic
Nov 22nd • 3 mins read

Comment on: Oncology research in Saudi Arabia over a 10-year period. A synopsis
Jun 24th • 3 mins read

The regulatory landscape of precision oncology laboratory medicine in the United States - Perspective on the past 5 years and considerations for future regulation
May 22nd • 8 mins read
